Pharma 4.0: Smarter asset management in pharma manufacturing
Posted: September 25, 2025

Most pharmaceutical companies recognize the need to invest in digital initiatives and advanced data analytics tools to remain competitive. However, the industry as a whole has been slow to move beyond isolated use cases to more holistic digital transformation. Fewer than one in five biopharma companies have effectively carried out a digital transformation—significantly lower than the cross-industry average of 35%.1 This lag is partly due to the challenges of introducing new digital tools in a heavily regulated industry.
According to the International Society for Pharmaceutical Engineering (ISPE), for digital transformation to deliver ROI, one thing companies must do is employ strategic and comprehensive asset management. From HVAC systems that keep pharma plants at the right temperature and humidity to machines that produce a company’s life-saving drugs, precision and efficiency in production are paramount. This is why organizations are looking at solutions that enable real-time data analysis for predictive maintenance and asset optimization to remain competitive.
Ensuring operational data is FAIR
Pharmaceutical manufacturing requires precise environmental and mechanical control. Even slight deviations in equipment performance (e.g. vibration, temperature, or pressure) can compromise drug quality or safety.
The first step in leveraging operational data to get medicines to market faster while maintaining safety and quality is making sure it is FAIR:
- Findable: Operators and analysts need to easily locate relevant data using standardized metadata and identifiers, without having to dig through multiple folders or systems.
- Accessible: Data needs to be securely accessible, enabling data sharing within and across teams, while maintaining compliance with regulations like GxP and ISO standards.
- Interoperable: Data should be formatted and structured so that different systems and software can understand and use that data. This breaks down silos and enables integration—for example, manufacturing data from sensors can be combined with lab results and quality data for more advanced analysis.
- Reusable: For data to have long-term value, it should be well-documented and trustworthy enough to be reused in new experiments or for AI models without needing to regenerate it from scratch.
Scientific data cloud enables bioreactor optimization
A good example of what this might look like is biotech company Vir Biotechnology, known for developing one of the first Covid-19 antibodies. Faced with siloed, hard-to-access bioreactor data, the company was finding it challenging to apply advanced analytics and AI for process optimization. Scientists were spending up to 30% of their time finding, cleaning, and duplicating data for each study—time that could be better spent improving processes and advancing medical innovation.
So, partnering with Cognizant, Vir implemented a “scientific data cloud” using CONNECT as the central data historian. Data from hundreds of instruments—including high-resolution bioreactor and chromatography system data—is now automatically ingested and accessible through a standardized, queryable data mesh.
With AVEVA’s advanced analytics, visualization, and Power BI integration, scientists gained real-time, shareable dashboards to monitor bioreactor operations, trend datasets, and receive automated alerts. This one-stop shop for all real-time bioreactor data halved the time spent on analysis and reporting, reduced errors, lowered IT costs, and created a scalable foundation for AI-driven predictive analytics as Vir advances its commercialization efforts.
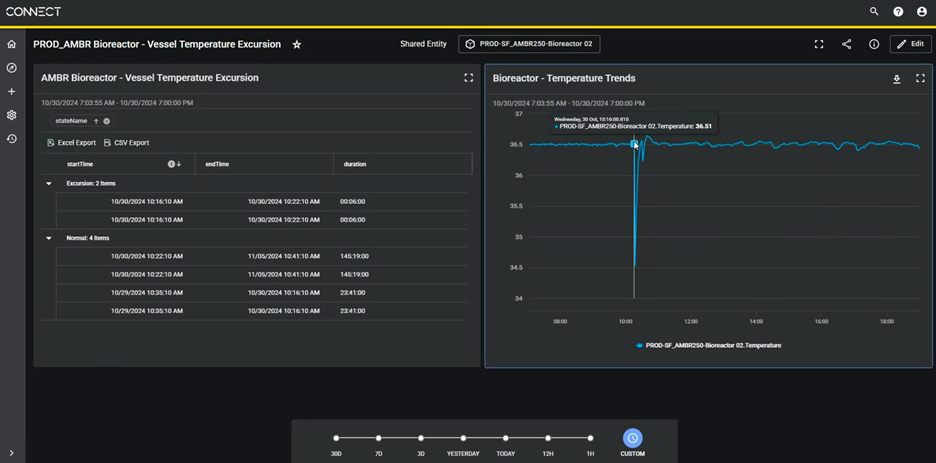 Using real-time shareable dashboards, users can monitor bioreactor operations, trend datasets, and receive automated alerts
Using real-time shareable dashboards, users can monitor bioreactor operations, trend datasets, and receive automated alerts
Using vibration sensors for comprehensive condition monitoring
The processes involved in producing a medication are complex to manage in a lab setting, and they become even more challenging in industrial production. Proactive and predictive equipment maintenance is essential for managing this production.
Bausch + Ströbel, a leading maker of pharmaceutical filling machines, and BK Vibro, an expert in vibration-based condition monitoring, have created a joint solution that uses AVEVA™ PI System™ and CONNECT to provide comprehensive condition monitoring to enhance the overall equipment efficiency (OEE) and reliability of pharmaceutical manufacturing operations.
This solution integrates BK Vibro’s state-of-the-art vibration sensors and the IoT gateway OMNIA Connect to streamline data to AVEVA PI System. Contextualized data is then transferred to CONNECT to use advanced analytics for anomaly detection. This integration allows for advanced condition monitoring, real-time data analysis, and predictive maintenance, ensuring optimal performance and reduced downtime for pharmaceutical manufacturing processes. A key feature of this solution is its ease of integration and the ability to retrofit to existing machines without requiring changes to qualified machines or PLCs.

Eli Lilly implements a scalable, flexible solution to monitor critical spaces
In addition to the criticality of monitoring the production equipment in a pharma plant, the management of building systems is vital to controlling the parameters of spaces. Factors such as temperature, humidity, and cleanliness of labs and plants are especially important in pharmaceutical manufacturing.
Eli Lilly, a leading pharmaceutical company that produces life-saving medicines for people all over the world, built a qualified building management system (QBMS) at one of its new sites in Ireland. The company wanted to be able to monitor critical parameters for controlled spaces like clean rooms, labs, and storage areas. And it needed a solution that was cost-effective and simplified its current automation stack.
Already an AVEVA PI System user, the team decided to take advantage of all the system has to offer with AVEVA™ PI Server’s Asset Framework, analytics, event frames, notifications, and AVEVA™ PI Vision™ dashboards. The result? Reduced complexity, minimized costs—the company saved 25% in system costs—and improved real-time monitoring of systems and spaces, all while ensuring compliance with the strict environmental standards of pharmaceutical manufacturing.

Advanced data management platform for efficiency and compliance
Bioprocessing is notoriously inefficient. As Tim Wortley from Cytiva, one of the world’s largest biotech equipment providers, says, “We always joke we’re twenty, thirty years behind plastics, oil and gas, you name it—a lot of time spent collecting data, nobody has central systems, a lot of times you just can’t find the data or it’s in paper records, batch runs have a high failure rate.”
SyVento, a new biotech company in Poland, was familiar with this kind of inefficiency. SyVento develops mRNA therapies, training the immune system to recognize and then kill tumor cells. One promising procedure includes collecting T cells and infecting these cells with a virus such as HIV that will genetically change them so they’ll see and react against cancer cells.
The bioprocessing required for the development of these therapies was challenged with siloed data and separate systems. So SyVento partnered with Cytiva to adopt a standard data platform. The FlexFactory™ historian, built on the AVEVA PI System, provides centralized data aggregation, visualization, and reporting for both new and existing biomanufacturing trains. The implementation of the FlexFactory historian at SyVento Biotech’s new mRNA manufacturing facility in Poland demonstrates significant improvements in efficiency.
In addition to efficiency gains, the new system ensures easy and accurate regulatory compliance, which is crucial in the pharmaceutical industry. As Tim Wortley says, “If it isn’t documented, it didn’t happen. Whether you have the most wonderful drug in the world—if you don’t have the paperwork, you might as well just throw it away. That data is sacrosanct. That data is the batch. That data is the drug.”
The FlexFactory provides a future-proofed and digitally enabled manufacturing platform to meet future regulatory expectations for data integrity and analysis, providing frictionless movement of data between manufacturers, client biopharmaceuticals, and regulators. This allows SyVento to deploy a quicker, smarter drug batch release, improving time to market and gaining a competitive edge.
The life sciences ecosystem: The next frontier
Vir Biotechnology is a research and development company focused on bringing new therapies to market. Eli Lilly is a tier-one pharmaceutical manufacturer. Cytiva and Bausch + Stroebel are both equipment suppliers. They are all part of the life sciences ecosystem supported by AVEVA solutions. The next frontier of operational efficiency and regulatory compliance in life sciences requires a collaborative effort among license holders, contract manufacturers, equipment vendors, and regulatory agencies. Connected ecosystems help organizations overcome silos in the value chain to ensure the success of a product across development, manufacturing, and regulatory compliance—and with secure, cloud-first data management solutions, these connected ecosystems can thrive into the future of pharma 4.0.
Digital transformation built on intelligent data platforms
The pharmaceutical industry is at a turning point, where embracing intelligent asset management and condition monitoring is no longer optional—it’s essential. With advanced data platforms, organizations can break down data silos, boost operational efficiency, and improve regulatory compliance. Real-world examples from companies like Vir Biotechnology, Eli Lilly, and SyVento demonstrate the tangible benefits of scalable, integrated solutions for both equipment and environmental monitoring. By making operational data FAIR and using advanced analytics, pharma companies can unlock new levels of productivity and innovation. As the industry continues to evolve, digital transformation backed by intelligent infrastructure will be the key to staying competitive and accelerating time to market.
1https://media-publications.bcg.com/BCG-Biopharma-Digital-Transformation-by-Industry-June-2022.pdf
Related blog posts
Stay in the know: Keep up to date on the latest happenings around the industry.